Home > Business license
Business Licenses for Medical Devices
Business licenses required to manufacture, import, wholesale, retail, rent or repair for Medical Devices in Japan are as listed below.
- Overseas Manufacturer
- Marketing Authorization Holder (MAH)
*DMAH (Designated MAH) is required to have this license. - Domestic Manufacturer (Warehouse of medical devices in Japan also needs to have this license)
- Retail/Rental Service of medical devices in Japan
- Repair of medical devices in Japan
(Note)
1 - 3: Licenses / registration required at the time of submission for approval
4 - 5: Licenses required after obtaining approval
The relationships linking the various licenses and a product flow are shown below.
*PMDA: Pharmaceuticals and Medical Devices Agency
*RCB: Registered Certification Body
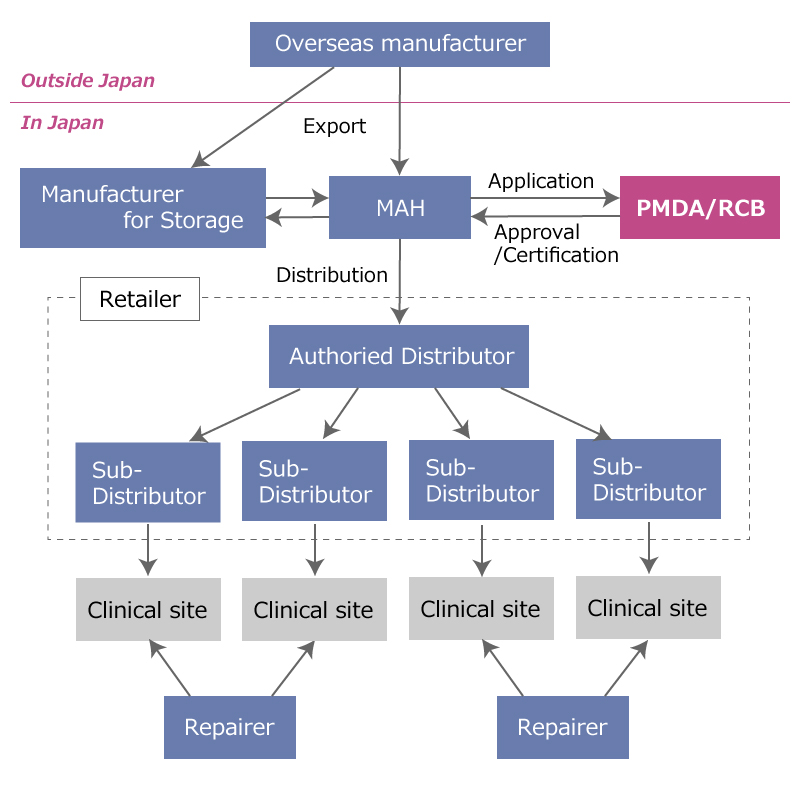
Fig 1: In case of MAH (Marketing Authorization Holder) company is applicant
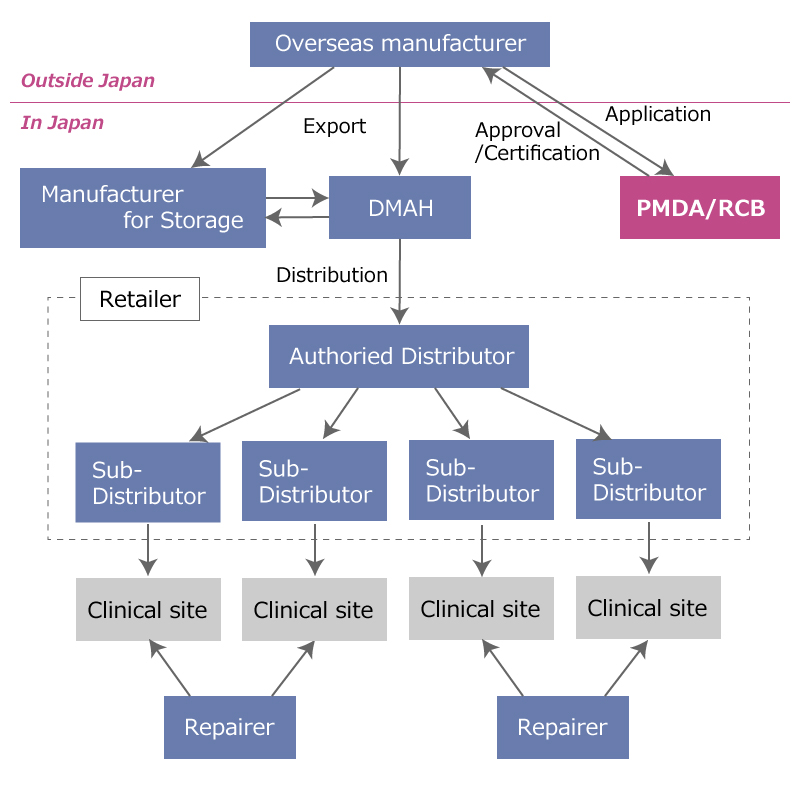
Fig 2: In case of overseas manufacturer is applicant
Manufacturer
Manufacturers of medical devices have to be registered as a medical device manufacturer, when a medical device manufactured at their facilities is registered in Japan.
Overseas manufacturers also have to be registered via a representative company in Japan.
Facilities in Japan where medical devices are stored are also regarded as “medical device manufacturers”. So such facilities have to be registered as manufacturers in Japan.
Marketing Authorization Holder (MAH/DMAH)
In Figure 1, when medical devices are registered and marketed in Japan, the MAH is the key organization.
In Figure 2, a DMAH (= Designated MAH) is required to act as a representative organization for the foreign manufacturer.
The requirements and tasks of MAH and DMAH are mostly the same.
There are three categories of MAH license, and medical devices that can be handled by the MAH depend on these categories as shown in the Table 1.
Table1: MAH classifications
| MAH classification | Scope |
|---|---|
| 1st-class MAH | Allowed to handle all classes of medical devices. |
| 2nd-class MAH | Allowed to handle Class I and Class 2 medical devices. |
| 3rd-class MAH | Allowed to handle Class I medical devices only. |
*MAH and DMAH
MAH (Marketing Authorization Holder):
This is the name of the license given to an organization that can act as an applicant/approval holder.
DMAH (Designated MAH):
This is not the name of the license. A company with a MAH license can become a DMAH to act as an organization representing a foreign manufacturer in its submission of an application for product registration. However, in this case, the approval holder is the foreign manufacturer.
Retail/Rental Service
Licenses for medical device retail/rental services are necessary for commercial sale or rental of medical devices.
The requirements and tasks related to the license depend on the medical device classification.
Repair Service
Medical device repairers must have a license of Medical device repairer.
Since required knowledge and skills differ depending on types of medical devices, there are 18 categories in medical device license. And applicable categories of license should be obtained.




